Brain-machine interface (BMI), or Brain-computer interface (BCI) establishes a direct communication and control channel between the brain and the external world, Figure 1. BMI technology greatly enhance human understanding of how the brain works and how to treat brain diseases. It has become the scientific and technological strategic focus or core technology development area of many countries.
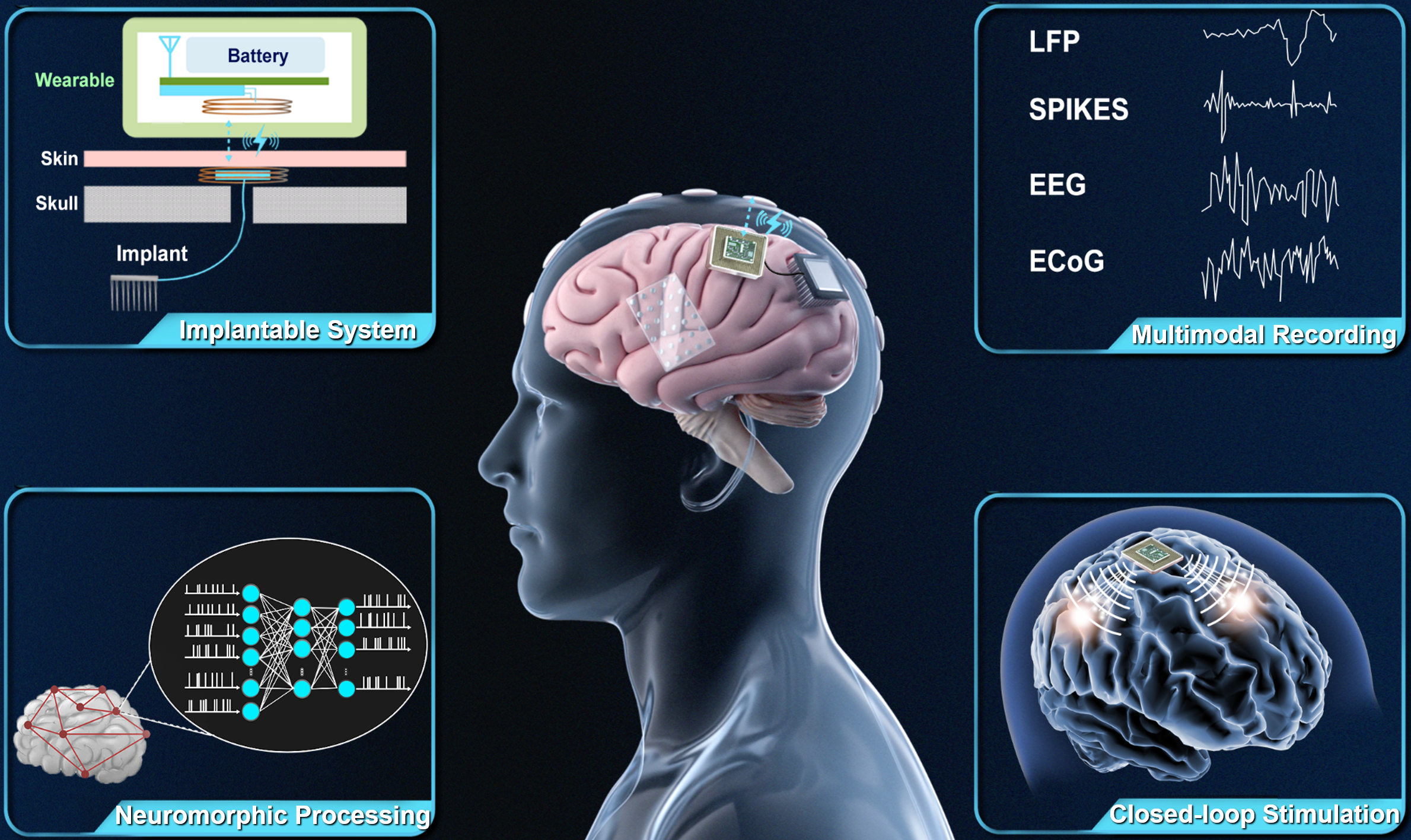
Figure 1. Brain-machine/computer interfaces.
Many physiological functions, such as sensory perception, movement, cognition, and emotion, are coordinated and controlled through electrical signals. By applying electrical/optical stimulation to some brain areas, lost physiological functions can be understood and restored. Electrical/optical stimulators are devices that serve such function of generation of desired stimuli. They are widely used for epilepsy control, addiction, depression treatment, Parkinson's disease, vision, etc. Besides direct electrical stimulation, optogenetics is an alternative methodology to excite or control neurons that have been genetically modified to express light-sensitive ion channels. In CenBRAIN, we use neural recording circuits and systems we accomplished to monitor the neuron activities and based on machine learning diagnose tools to decide how and when a stimulation should be generated to enhance the physiological functions [1].
Biocompatibility and flexibility of electrodes are key issues for implantable brain-machine/computer interface applications. We study in CenBRAIN biocompatible materials of electrodes to avoid chronic inflammatory response at the electrodes-tissues’ interfaces and the surrounded sites. In addition, we propose flexible microelectrodes to avoid mechanical mismatch with the surrounded tissues. Among the introduced electrodes in our group, the cuff format ones including Shape memory alloy (SMA) which are designed to facilitate connecting to sacral root peripheral nerves in a bladder controller. Also, 3D pyramid-shaped silicon-based microelectrode arrays where their tips are covered by carbon nanotube to minimize the stimulation current density. Moreover, porous and notched mini-grid electrodes are made for wireless intracranial electroencephalographic recordings. Still, pin-shaped conductive polymer electrodes are fabricated for electrocardiogram (ECG) and electroencephalographic (EEG) recordings.
Wearable and implantable medical devices may use batteries, however the latter have limited energy budgets which make continuous operation of such devices impossible. Wireless techniques can be used to recharge the batteries or to directly recover energy to avoid battery. In fact, energy can be harvested from solar, body motion, radiofrequency, etc. Different types of transducers can be used to harvest the mechanical energy of the body including electrostatic, electromagnetic, piezoelectric, triboelectric, and thermoelectric devices. These harvesters are capable of producing sufficient energy for implantable or wearable devices, but they are bulky. Radio Frequency (RF) energy harvesting is an approach in which ambient RF signals are converted into electrical energy by using antennas. Assuming that wireless communication signals are available everywhere, this type of harvester is small and can be integrated with the biomedical device, but there is still the issue of low efficiency of the RF-to-DC converter.
Wireless power transfer (WPT) is a technology which can transmit energy and data from one site to another site wirelessly, as shown in Figure 2. It can be implemented through inductive link, capacitive link, ultrasonic link, where the inductive link is the most efficient method. An external coil and internal coil make up an inductive link to implement WPT and wireless bidirectional communication. The external device is used to sense the status of the implantable (or receiving) part, send it the energy through inductive link to activate (or to wake up), and control the internal device through same inductive link as well. Then the internal device recovers the data and generates the stimuli in current mode to the electrodes, implementing the electrical stimulation process. Also, the implant measures parameters and transmits back to the external device through the same inductive link, the external device adjusts the intensity of the stimulus instantly according to the measurement information through this implemented closed-loop monitoring/recording and stimulation system.
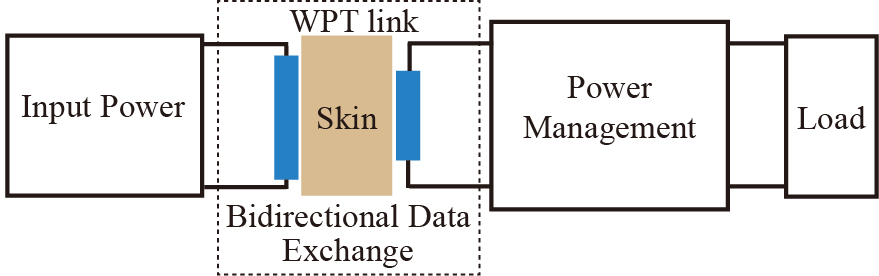
Figure 2. Wireless power transfer (WPT) technique.
According to the quantitative evaluation of the World Health Organization, brain diseases account for 28% of the public health burden of various diseases on the society. It has exceeded cardiovascular diseases or cancer and became the one of the primary threats to human health. Currently, the causes of various types of brain diseases are still unknown, and there is a lack of effective treatment measures. Currently, early diagnosis and early intervention is the most effective medical treatment for these brain diseases.
In CenBRAIN, we adopt advanced artificial intelligence algorithms to achieve accurate prediction and diagnosis of brain diseases such as epilepsy [2-3]. Moreover, dedicated low power artificial intelligence architectures and circuits have been developed to enable wearable, implantable devices with intelligence to handle complex prediction tasks, personalized adaptation, and online learning.
[1] G. Rong, A. Mendez, E. Bou Assi, B. Zhao, and M. Sawan, "Artificial Intelligence in Healthcare: Review and Prediction Case Studies," Engineering, 2020/01/03/ 2020, doi: 10.1016/j.eng.2019.08.015.
[2] J. Yang and M. Sawan, "From Seizure Detection to Smart and Fully Embedded Seizure Prediction Engine: A Review," IEEE Transactions on Biomedical Circuits and Systems, vol. 14, no. 5, pp. 1008-1023, 2020, doi: 10.1109/TBCAS.2020.3018465.
[3] Z. Wang, J. Yang, H. Wu, J. Zhu, and M. Sawan, “Power efficient refined seizure prediction algorithm based on an enhanced benchmarking,” Scientific Reports, vol. 11, no. 1, pp. 23498, 2021/12/06, 2021.