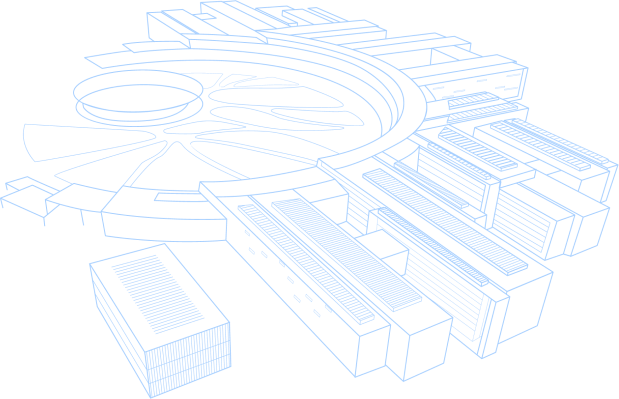
Virology and Immunology: Optical Biosensing and Vaccine Evaluation
Global pandemics emphasize the urgency for biosensor development to monitor outbreaks [1,2] while understanding antibody response to booster inactivated vaccines is crucial for global vaccination efforts to treat pandemics.To monitor infectious diseases, we designed an optical fiber biolayer interferometry (FO-BLI) based biosensor (Fig. 1A) to detect the emerging SARS-CoV-2 antigens [3] and the latest Monkeypox virus [4] within 14 mins. A silica-binding protein was designed to facilitate the site-directed antibody immobilization onto silicasurfaces (Fig. 1B). For a novel metacavity biosensor, ultra-high-Q (> 4000) along with high refractometric sensitivity (~ 450nm/RIU) was achieved. Notable limit-of-detection (~100 viral copies/mL) was observed for label-free SARS-CoV-2 detection, showing remarkable performance in bulk and surface sensing (Fig. 1C) [5]. Besides, Porous silicon-Tamm Plasmon Polariton biosensor based on Distributed Bragg Reflector coated with Au thin film is developed for real-time monitoring of antigen-antibody binding in a scanning process (Fig. 1C) [6]. To evaluate vaccine efficacy, we reported two FO-BLI biosensors for detecting SARS-CoV-2 neutralizing antibodies (NAbs) and binding antibodies(BAbs)in human serum [7] (Fig. 1C), within 7.5 and 13 min, respectively, detecting as low as 10 ng/mL. FO-BLI showed similar NAbs levels in microsamples and sera and correlated well with pseudovirus neutralization test [8]. For both topics, we applied machine learning to predict optical biosensing performance and the antibody levels over time [8, 9].
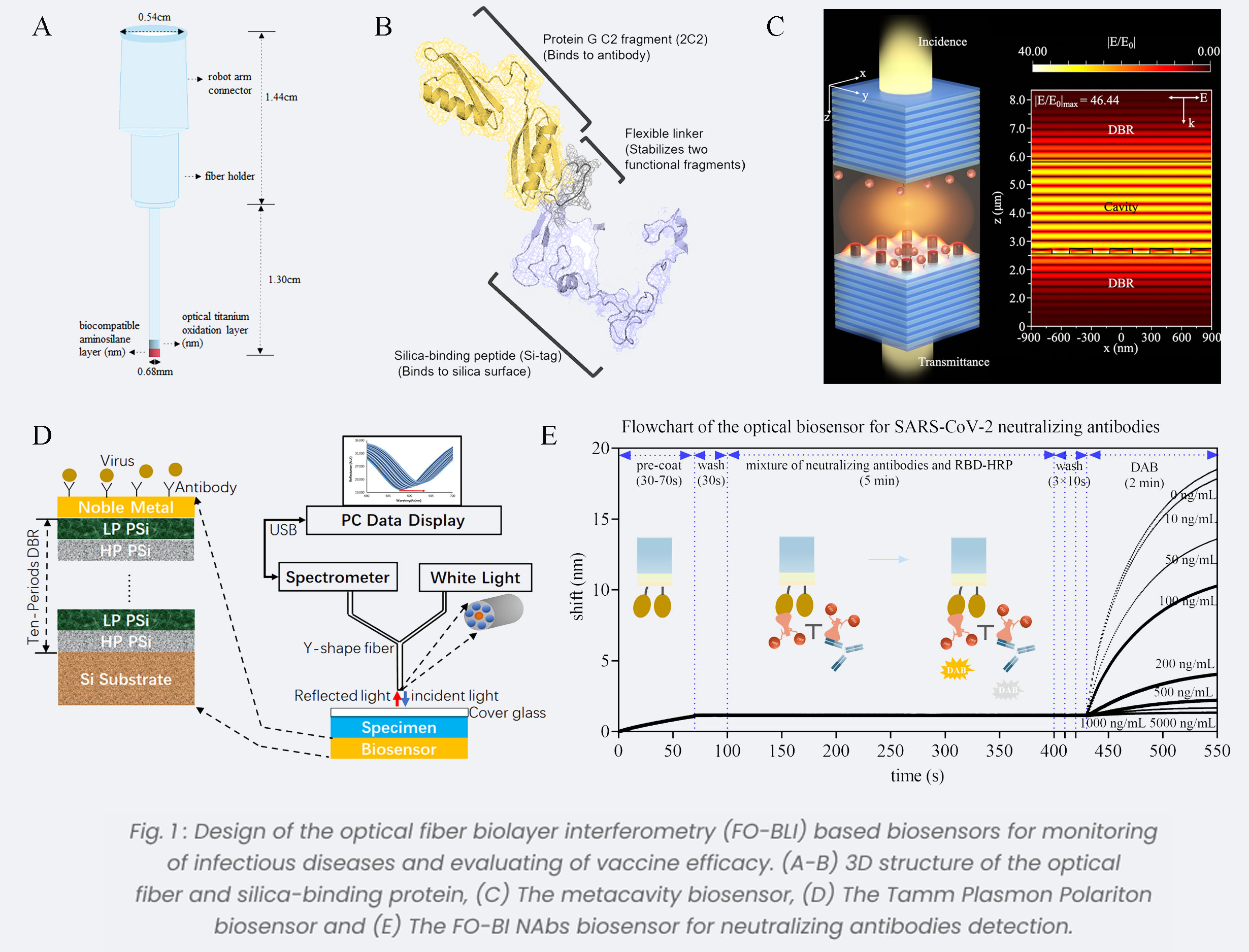
Neurological Disorders: Biosensing Technologies
Neurological disorders pose a significant burden on global disability-adjusted life-years. The emergence of biosensing platforms, integrating optical MEMS, electrochemical biosensors, and memristors, marks a new era of heightened sensitivity and precision in monitoring[10,11]. Optical MEMS excel in highly accurate, while electrochemical biosensors offer real-time biomarker quantification. Memristors enhance data processing efficiency. This integration promises transformative healthcare advancements, revolutionizing neurological disorder detection and management.In addressing Alzheimer's disorder (AD), we introduced innovative optical BioMEMS sensors for AD biomarker evaluation (Fig.2A). Through meticulous simulation and streamlined fabrication processes, our platform nears implementation, poised for early AD detection. Ongoing validation efforts using artificial and real samples enhance diagnostic capabilities. Additionally, our highly sensitive electrochemical biosensors enable real-time monitoring of neurotransmitters, fostering a deeper understanding of neurotransmission dynamics and facilitating neurological disorder diagnosis and treatment (Fig.2B). These platforms enable direct monitoring from living cells, enriching our understanding and enabling targeted interventions for neurological disorders[12]. Furthermore, we proposed an advanced memristor-based biosensor array for ultra-sensitive neurochemical detection[13]. Fabricated TiO2-based memristive cells exhibit exceptional electric field and efficient switching at remarkably low voltages (Fig.2C). Rigorous testing ensures the reliability and longevity of our biosensor array, offering transformative potential in neurochemical analysis. An I-V curve is shown in Fig.2D for the first test that involved grounding BE (Au) and applyinga voltage to TE (Pt). Also, we conducted over 2000 cycle tests to determine the stability of our device, Fig.2E illustrates HRS and LRS results.
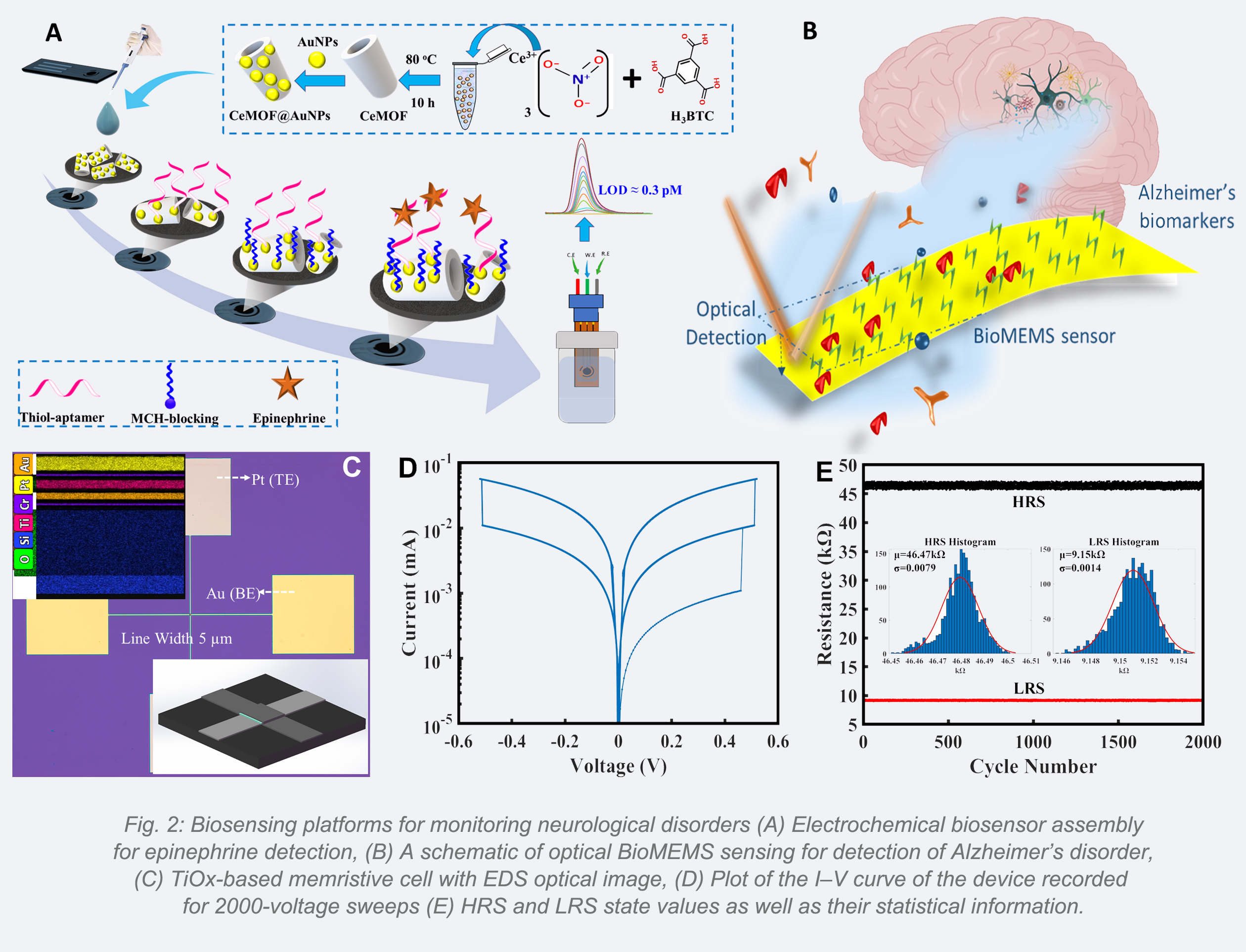
Single Neuron: Determination and Manipulation
Accurate monitoring of small molecules and precise characterization of the secretory ability of neurons are essential for effective diagnosis of neurological diseases. Chips-based platforms intended for single-cell manipulation are powerful tools to analyze intercellular interactions[14]. While conventional cell co-culture models can provide insights into cell communication to some extent, understanding the role of single cell requires further analysis. We have discovered that trapping of small molecules by aptamers induces a conformational change, altering the nanostructure of irregularly shaped Au nanoclusters and aptamers [15] due to the strong structure-property relationships of Au NC-aptamers (Fig.3A). This change can be detected through changes in resonance Rayleigh scattering intensity in the dark field. This new technology addresses challenges related to precise control of individual nanoparticles and the difficulty of small spectral peak shifts. Additionally, we have designed and fabricated a small-scale microelectrode array MEA chip to manipulate single cells and investigate intercellular connections in vitro [16]. A non-uniform electric field is generated in microfluidic channels to trap cells,facilitating precise cell positioning for real-time monitoring of their growth and intercellular interactions. Fig.3B depicts the simplified illustration of the proposed device.
Organoids-on-chip: Neurodegenerative and Inflammatory Bowel Disease
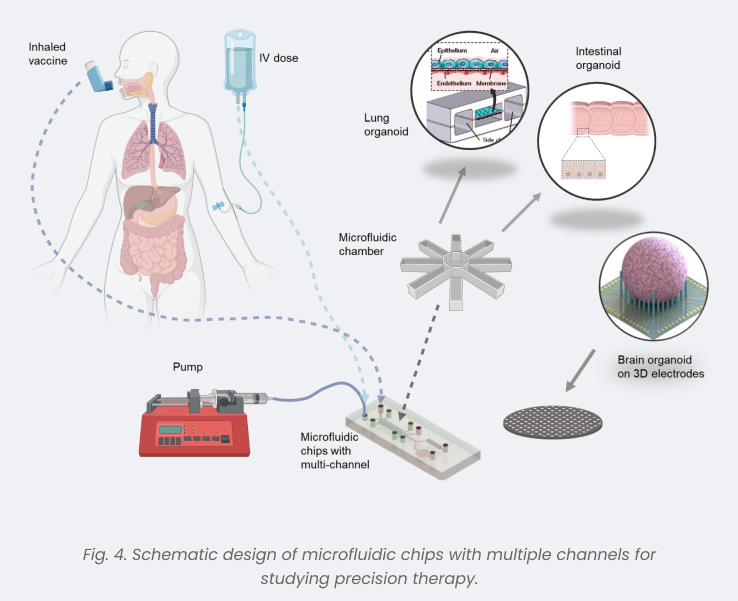
The current landscape of in vitro cell culture and animal models falls short in replicating the physiological intricacies of human organs. Enter Organoids, heralded as the new gold standard poised to redefine disease modeling, drug development, and precision medicine. With their superior physiological relevance and enhanced throughput,organoidsoffer promising avenues for research and application. Nevertheless, challenges persist, notably hypoxia within cell clusters and the complexity of multi-organ co-culture.To address these hurdles, our proposal outlines the establishment oforganoids-on-chip (OoC)models specifically engineered to mitigate hypoxia within cell clusters. Leveraging a modular microfluidic chip platform, we aim to simulate and optimize multi-organ co-culture scenariosandexplorethe feasibility of culturing immune cells with organoids to further enhance the fidelity of in vivo microenvironments(Fig. 4). Additionally, we will pioneer experimental models to evaluate the safety and efficacy of noveldrug/biologicalcandidates.Within our center, we prioritize investigations into two pivotal areas: neurodegenerative diseases[14]and inflammatory bowel disease[17, 18]. Through rigorous examination of disease mechanisms and therapeutic candidates, we aim to pioneer personalized medicine approaches tailored to individual patient needs.This work is undersupportbyWestlake University Industries of the Future Research Funding.
References
[1]
Y. Zheng, X. Song, Z.Fredj, S. Bian, M. Sawan. "Challenges and perspectives of multi-virus biosensing techniques: A review," Analyticachimicaacta, 2023.[2]
X. Song, Z.Fredj, Y. Zheng, H. Zhang, G. Rong, S. Bian, M. Sawan. "Biosensors for waterborne virus detection: Challenges and strategies," Journal of pharmaceutical analysis, 2023.[3]
Y. Tao, S. Bian, P. Wang, H. Zhang, W. Bi, P. Zhu, M. Sawan. "Rapid Optical Biosensing of SARS-CoV-2 Spike Proteins in Artificial Samples," Sensors (Basel, Switzerland), 2022.[4]
X. Song, Y. Tao, S. Bian, M. Sawan.“Optical Biosensing of Monkeypox Virus Using Novel Recombinant Silica-Binding Proteins for Antibody Site-Directed Antibody Immobilization,” J. Pharm. Anal. under revision, 2024.[5]
Y. Zheng, S. Bian, J. Sun, L. Wen, G. Rong, M. Sawan. "Label-Free LSPR-Vertical Microcavity Biosensor for On-Site SARS-CoV-2 Detection," Biosensors, 2022.[6]
G. Rong, Y. Zheng, X. Li, M. Guo, Y. Su, S. Bian, B. Dang, Y. Chen, Y. Zhang, L. Shen, H. Jin, R. Yan, L. Wen, P.Zhu, M. Sawan. "A high-throughput fully automatic biosensing platform for efficient COVID-19 detection," Biosensors & bioelectronics, 2023.[7]
S. Bian, M. Shang, M. Sawan. "Rapid biosensing SARS-CoV-2 antibodies in vaccinated healthy donors," Biosensors & bioelectronics, 2022.[8]
S. Bian, M. Shang, Y. Tao, P.B. Wang, Y.K. Xu, Y. Wang,Z..DShen, M. Sawan. “Dynamic Profiling and Prediction of Antibody Response to SARS-CoV-2 Booster-Inactivated Vaccines by Microsample-Driven Biosensor and Machine Learning,” Vaccines, 2024.[9]
G. Rong, A. Mendez, E. Bou Assi, B. Zhao, M. Sawan. "Artificial Intelligence in Healthcare: Review and Prediction Case Studies," Engineering, 2020.[10]
F. Marvi, Y. H. Chen, M. Sawan. "Alzheimer's Disease Diagnosis in the Preclinical Stage: Normal Aging or Dementia," IEEE reviews in biomedical engineering, 2024.[11]
Z.Fredj, M. Sawan. "Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends," Biosensors, 2023.[12]
Z.Fredj, M. Ali, B. Singh,E. Dempsey. "Simultaneousvoltammetricdetection of 5-hydroxyindole-3-acetic acid and 5-hydroxytryptamine using a glassy carbon electrode modified with conducting polymer andplatinisedcarbon nanofibers," Mikrochimicaacta, 2018.[13]
F. Ullah, M. Tarkhan, Z.Fredj, Y. Su, T. Wang, M. Sawan. “A Flexible High-Accuracy Memristor Model Using Varactors,” Nano Ex, 2023.[14]
H. Zhang, G. Rong, S. Bian, M. Sawan. "Lab-on-Chip Microsystems for Ex Vivo Network of Neurons Studies: A Review," Frontiers in bioengineering and biotechnology, 2022.[15]
Y. Su, G. Rong, S. Bian, P. Wang, L. Li, Y.-H. Chen, C. Huang, H. Zhang, M. Sawan. "Dark‐Field Resonance Rayleigh Scattering Biosensor to Monitor Small Molecules and Determine the Secretory Ability of Single Neuron," Advanced Materials Technologies, 2024.[16]
H. Zhang, P. Wang, N. Huang, L. Zhao, Y. Su, L. Li, S. Bian, M. Sawan. "Single neurons on microelectrode array chip: manipulation and analyses," Frontiers in bioengineering and biotechnology, 2023.[17]
S. Bian, E. Dreesen, H. T. Tang, G.Compernolle, M. Peeters, G. Van Assche, M. Ferrante, S. Vermeire,A. Gils. "Antibodies Toward Vedolizumab Appear from the First Infusion Onward and Disappear Over Time," Inflammatory bowel diseases, 2017.[18]
S. Bian, M. Ferrante,A. Gils. "Validation of a Drug-Resistant Anti-Adalimumab Antibody Assay to Monitor Immunogenicity in the Presence of High Concentrations of Adalimumab," The AAPS journal, 2017.