Our research was published as a cover article in Advanced Materials technologies.
Titled “Dark-Field Resonance Rayleigh Scattering Biosensor to Monitor Small Molecules and Determine the Secretory Ability of Single Neuron”, this contribution has been published in the Advanced Materials Technologies. This article discovers that the trapping of small molecules to aptamers induces a conformational change that alters the nanostructure of irregularly shaped Au nanoclusters (NCs) and aptamers due to the strong structure-property relationships of Au NC-aptamers.
Congratulations to Yi Su and to this paper’s co-authors for this innovative achievement.

Reference:
Su, Y., Rong, G., Bian, S., Wang, P., Li, L., Chen, Y. H., ... & Sawan, M. (2024). Dark‐Field Resonance Rayleigh Scattering Biosensor to Monitor Small Molecules and Determine the Secretory Ability of Single Neuron. Advanced Materials Technologies, 2301701.
More information can be found at the following link:
https://onlinelibrary.wiley.com/doi/abs/10.1002/admt.202301701
Research Highlights:
1.The conformational change of aptamer caused by the binding of small molecules induces changes in the effective radius of the Au NCs-aptamer complex because of their strong structure-property relationships.
2.An easy fabrication of a large sensing area sensor employing the average effect of the electromagnetic field (EM) of the effective radius of irregularly shaped Au NCs-aptamer and further designed a sensing platform to work in the dark-field. It overcomes the precise control of interparticle distance, particle shape and morphology, and the change of EM intensity shows a better performance compared with the small spectral peak shift when detecting molecules with small refractive index changes.
3.This sensing platform can show the different concentrations of dopamine, which were secreted from single neurons of the seizure model and healthy neurons after polarizationusing a 633-nm laser.
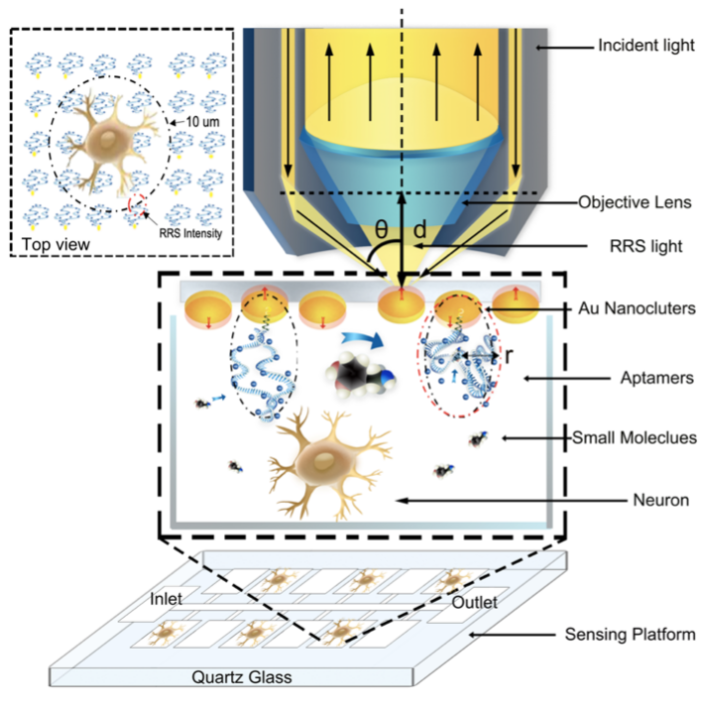
Fig.1: Schematic of the RRS device for monitoring the neurotransmitters released from neurons and incident light, and we monitor the RRS ΔI/I at the point of the red circular, which diameter is 1 µm in the top view (RRS, resonance Rayleigh scattering, ΔI/I, changing intensity versus original intensity).
Abstract:
Accurate monitoring of small molecules and precise characterization of the secretory ability of neurons are essential for effective diagnosis of neurological diseases. However, existing technologies for monitoring small molecules only provide approximate results. Plasmonic-based biosensors are a potential solution in this biological application. Nevertheless, optical sensing nanoparticles require precise control of interparticle distance, particle shape and morphology, which can be challenging. In this article, we discover that the binding of small molecules to aptamers induces a conformational change that alters the effective radius of irregularly shaped Au nanoclusters (NCs)-aptamer complexes due to the strong structure-property relationships of these complexes. This change can be detected via resonance Rayleigh scattering (RRS) intensity in the dark-field. This new technology overcomes the challenges of precise control of individual nanoparticles and the difficulty of small spectral peak shifts. The Au NCs-aptamer sensing platform has a detection limit of 0.112 pM for dopamine, and we detect 0.226 pM of dopamine surrounding a single epileptic neuron in polarization, which is more than those of healthy neurons. Overall, this easy-to-fabricate Au NCs-aptamer-based technology has a high potential for monitoring small molecules and determining the secretory ability of single neurons, which is not possible using traditional technologies.
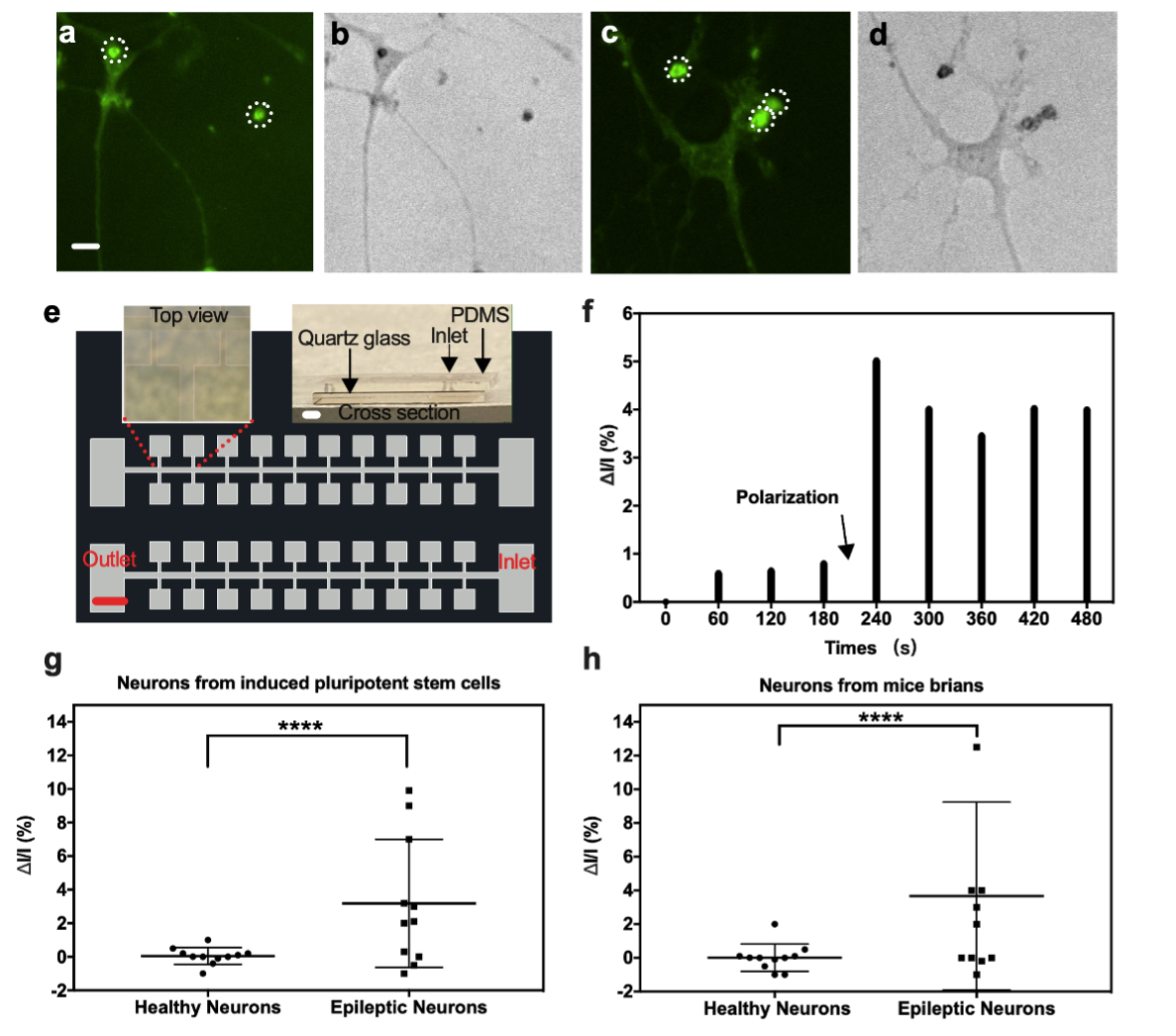
Fig. 2: Determination of secretory ability of neurons: Stimulation the single healthy neuron in embedding fluorescent probes a) under fluorescence microscopy and b) under bright‐field microscopy, Stimulation the single epileptic neuron in embedding fluorescent probes c) under fluorescence microscopy and d) under bright‐field microscopy (The large green dot in white dot line is background noises, scale bar: 20 µm), e) Optical microscopy of microfluid chip with microarrays and cross‐section of the biosensor (scale bar = 500 µm both for microfluid chip and cross‐section of biosensor), f) Dark‐field RRS ΔI/I response of the biosensor detecting dopamine released from the epileptic neurons from mice brains with time, g) Dark‐field RRS ΔI/I of dopamine released from healthy neurons versus epileptic neurons from induced pluripotent stem cells, h) Dark‐field RRS ΔI/I of dopamine released from healthy neurons versus epileptic neurons from mice brains. Error bars in (c) and (d) are ± SD with N = 11 samples per group. ****p value < 0.0001 healthy neurons versus epileptic neurons in (g) and (h), (RRS, resonance Rayleigh scattering, ΔI/I, changing intensity versus original intensity).