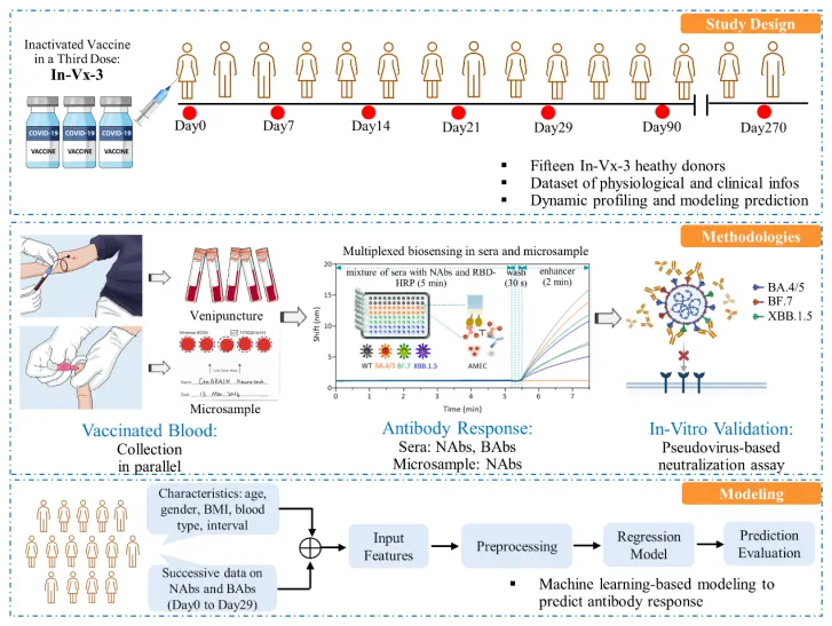
Titled “Dynamic Profiling and Prediction of Antibody Response to SARS-CoV-2 Booster-Inactivated Vaccines by Microsample-Driven Biosensor and Machine Learning”, this contribution has been published by Vaccines.
Congratulations to Dr. Bian, Dr. Shang and other CenBRAIN Neurotech members for this excellent achievement!
Citation
Bian S, Shang M, Tao Y, Wang P, Xu Y, Wang Y, Shen Z, Sawan M. Dynamic Profiling and Prediction of Antibody Response to SARS-CoV-2 Booster-Inactivated Vaccines by Microsample-Driven Biosensor and Machine Learning.Vaccines. 2024; 12(4):352.
More information can be found in this link:
https://www.mdpi.com/2076-393X/12/4/352
Abstract
Knowledge of the antibody response to the third dose of inactivated SARS-CoV-2 vaccines is crucial because it is the subject of one of the largest global vaccination programs. This study integrated microsampling with optical biosensors to profile neutralizing antibodies(NAbs) in fifteen vaccinated healthy donors, followed by the application of machine learning to predict antibody response at given timepoints. Over a nine-month duration, microsampling and venipuncture were conducted at seven individual timepoints. A refined iteration of a fiber optic biolayer interferometry(FO-BLI) biosensor was designed, enabling rapid multiplexed biosensing of the NAbs of both wild-type and Omicron SARS-CoV-2 variants in minutes. Findings revealed a strong correlation (Pearson r of 0.919, specificity of 100%) between wild-type variant NAb levels in microsamples andsera. Following the third dose, sera NAb levels of the wild-type variant increased 2.9-fold after seven days and 3.3-fold within a month, subsequently waning and becoming undetectable after three months. Considerable but incomplete evasion of the latest Omicron subvariants from booster vaccine-elicited NAbs was confirmed, although a higher number of binding antibodies (BAbs) was identified by another rapid FO-BLI biosensor in minutes. Significantly, FO-BLI highly correlated with a pseudovirus neutralization assay in identifying neutralizing capacities (Pearson r of 0.983). Additionally, machine learning demonstrated exceptional accuracy in predicting antibody levels, with an error level of <5% for both NAbs and BAbs across multiple timepoints. Microsample-driven biosensing enables individuals to access their results within hours of self-collection, while precise models could guide personalized vaccination strategies. The technology’s innate adaptability means it has the potential for effective translation in disease prevention and vaccine development.