Chair professor Mohamad Sawan's research center has recently published a paper in early lung cancer detection on the journal ACS Applied Materials & Interfaces. This study, conducted by Westlake University's CenBRAIN Neurotech Center of Excellence, successfully pushed the detection limits of a key biomarker for small-cell lung cancer, offering an innovative technological solution for early diagnosis of related diseases.

Neuron-specific enolase (NSE) is an acidic protease widely used as a biomarker for small-cell lung cancer and neuronal injury. Accurate detection of NSE is crucial for early diagnosis, prompting efforts to achieve extremely low detection limits. However, many current methods are costly, complex, and prone to amplification bias.
To address these challenges, our center introduces a novel electrochemical immunosensorfor ultrasensitive and specific NSE detection. This sensor employs AuNPs@CuMOF nanocomposites for both surface functionalization and signal amplification. Experimental results demonstrate remarkable detection sensitivity reaching 0.02 pg/mL for NSE, outperforming previously reported NSE biosensors. Furthermore, its performance in spiked serum samples correlates well with commercial ELISA results, highlighting its strong potential for reliable and rapid clinical diagnostics.
This study featured Dr. Mohamed Bahri as first author and Chair Professor Mohamad Sawan as corresponding author. The research received support from Westlake University, the Zhejiang Leading Innovative and Entrepreneur Team Introduction Program (Grant 2020R01005), and the Research Center for Industries of the Future at Westlake University.
Reference:
Bahri M, Rong G, Song X, Qin P, Sawan M. Synergistic AuNPs@CuMOF for Electrochemical Amplified Detection of Neuron-Specific Enolase. ACS Appl Mater Interfaces. 2025 Oct 15;17(41):56890-56901.
More information can be found at the following link: https://pubs.acs.org/doi/10.1021/acsami.5c16359
Research Highlights
1) A dual-purpose AuNPs@CuMOF design is used simultaneously for surface functionalization and signal amplification, simplifying biosensor fabrication.
2) Ultra-sensitive detection of NSE biomarker with a limit of detection (LOD) of 0.02 pg/mL across a wide range (0.1 pg/mL–200 ng/mL).
3) Reliable NSE detection in spiked human serum with a relative error between 5.63% and 12.03%, comparable to the ELISA detection method.
Abstract
Signal amplification has become a paramount strategy in biosensing, offering substantial improvements in sensitivity and detection limits for analytical platforms. However, conventional methods often rely on separate amplification stages and intricate chemical functionalization, leading to inefficiencies and greater complexity in biosensing workflows.
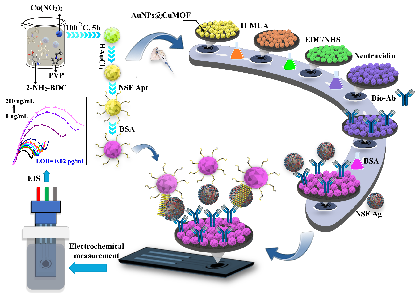
Figure 1: Illustration of the Functionalization Process and Working Flow of the Developed AuNPs@CuMOF-Based NSE Biosensor.
图1: 所开发的基于AuNPs@CuMOF的NSE生物传感器的功能化过程与工作流程示意图。
In this article, we introduce a novel dual-purpose application of AuNPs@CuMOF for both surface functionalization and signal amplification, enabling electrochemical biosensing of neuron-specific enolase (NSE), a key biological marker for neuronal disorders and small cell lung cancer. The proposed biosensing strategy implies immobilizing the biotinylated NSE antibody onto the AuNPs@CuMOF-functionalized surface of a screen-printed carbon electrode (SPCE), alongside a thiolated NSE aptamer conjugated to AuNPs@CuMOF for signal amplification. Following this approach, the proposed immunosensor showcased a detection limit (LOD) down to 0.02 pg/mL within the span of 0.1 pg/mL to 200 ng/mL, outperforming or matching previously reported NSE biosensors.Besides, the suggested NSE biosensor reliably identified NSE in spiked human serum samples, showing both strong selectivity and sensitivity, with performance metrics on par with those of standard ELISA tests, with the relative error ranging from 12.03% to 5.63%.
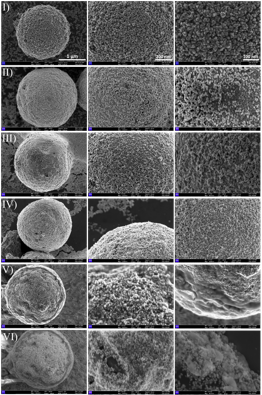
Figure 2: FESEM imaging attempts depict the morphological changes of AuNPs@CuMOF (I) and after each functionalization step: 11-MUA modification (II), EDC/NHS activation (III), NeutrAvidin immobilization (IV), biotinylated antibody attachment (V), and BSA blocking (VI).
图2: FESEM图像展示了AuNPs@CuMOF的形貌变化,以及每个功能化步骤后的形态:(I) AuNPs@CuMOF原始材料;(II) 经11-MUA修饰后;(III) 经EDC/NHS活化后;(IV) NeutrAvidin固定后;(V) 生物素化抗体附着后;(VI) BSA封闭后。
By leveraging the combined advantages of CuMOF’s large surface area, versatility, and stability, the proposed method simplifies the biosensing workflow while enhancing sensitivity through intrinsic amplification, resulting in a robust and efficient platform for the detection of biomarkers. We highlight in this work the transformative potential of multifunctional nanomaterials in advancing electrochemical biosensing technologies, which represents a significant milestone in tumor diagnosis in clinical settings.